Progeria Research Foundation Announces Collaboration and Supply Agreement with Eiger BioPharmaceuticals

Progeria Research Foundation Announces Collaboration and Supply Agreement with Eiger BioPharmaceuticals
FDA Guidance on Lonafarnib Approval for Progeria Sought
Children with Ultra-Rare, Fatal, Rapid-Aging Disease Die
of Heart Disease at an Average Age of 14 Years
PEABODY, MA, May 16, 2018 — The Progeria Research Foundation (PRF) announced a collaboration and supply agreement with Eiger BioPharmaceuticals for the development and pursuit of U.S. Food and Drug Administration review and potential approval of lonafarnib for the treatment of Progeria in children. Lonafarnib will mark the first therapy submitted to the FDA for the treatment of Progeria.
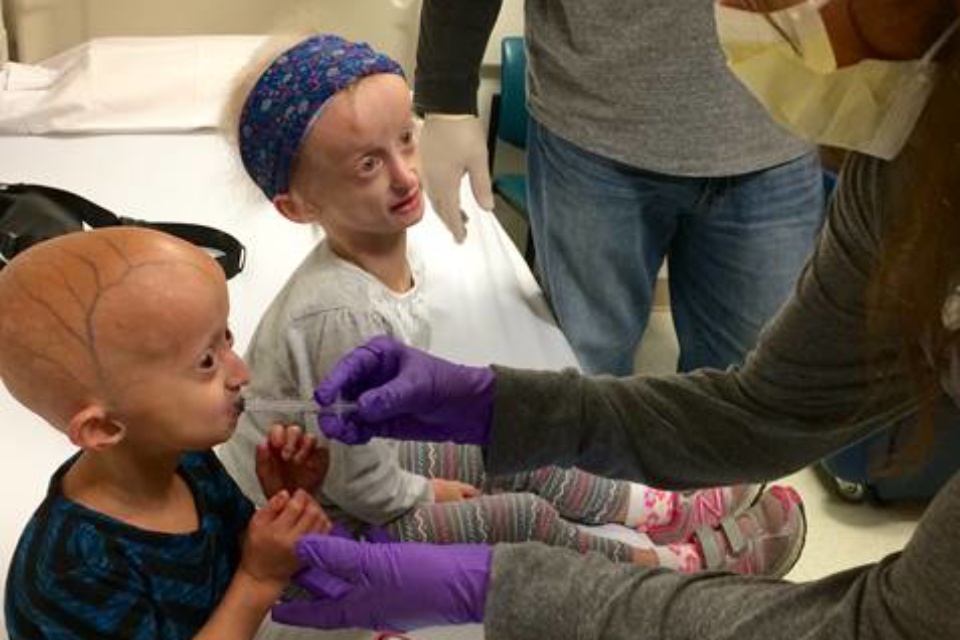
Zoey (L) and Carly (R) receive lonafarnib through their participation in ongoing clinical trials studying Progeria at Boston Children’s Hospital, funded by The Progeria Research Foundation.
2016 Photo, Courtesy of The Progeria Research Foundation
Occurring in approximately 400 children worldwide, Progeria is caused by a genetic mutation that results in an overabundance of the protein named progerin. Following PRF’s co-discovery of this mutation, the Foundation funded additional studies showing that, while progerin accumulation within blood vessels is typically seen in normal aging, progerin accumulation is highly accelerated in Progeria, causing progressive cellular damage and atherosclerosis in childhood. Without treatment, children with Progeria die of heart disease at an average age of 14.5 years.
Lonafarnib inhibits farnesyltransferase, an enzyme that facilitates progerin production. As a farnesyltransferase inhibitor (FTI), lonafarnib helps prevent cellular damage caused by the mutant protein. Lonafarnib is not currently approved for use outside of clinical trials. Since 2007, PRF has funded four clinical trials to study lonafarnib’s effect on Progeria, treating children from more than 30 countries.
PRF Medical Director Leslie Gordon, MD, PhD, along with her research teams at Boston Children’s Hospital and Brown University, recently published results of a study of lonafarnib in Progeria in The Journal of the American Medical Association (JAMA). The study reported that treatment with lonafarnib alone compared with no treatment was associated with a significantly lower mortality rate (3.7% vs. 33.3%) after a median of 2.2 years of follow up (see JAMA citations below).
“This progress is due in large part to the courage and strength of children with Progeria and their families who participated in the lonafarnib clinical trials,” said Dr. Gordon. “More than a decade of intensive investigation has enabled PRF to strike this partnership with Eiger and move towards potential FDA review and approval. Reaching this tremendous milestone gives us even more optimism that we will discover additional treatments that complement the lonafarnib therapy and keep us moving forward towards more effective treatments and the cure.” As the parents of a child with Progeria, Dr. Gordon and her husband, Scott Berns, MD, MPH, co-founded PRF in 1999 with Gordon’s sister Audrey Gordon, Esq.
Merck, known as MSD outside of the United States and Canada, previously provided lonafarnib free of charge for use in PRF-sponsored clinical trials. Following a transfer of manufacturing technology from Merck in 2015, Eiger began to provide lonafarnib at no cost for use in ongoing clinical trials funded by PRF. Eiger has since expanded its licensing agreement with Merck to include rights to develop lonafarnib for the treatment of Progeria and will be responsible for regulatory execution, commercialization and distribution activities across the licensed and approved indications in the future. PRF has granted Eiger a non-exclusive, world-wide license to all PRF intellectual property, know-how and clinical data generated by PRF to prepare and file a new drug application (NDA) for Progeria. Eiger also intends to establish an expanded access program that would enable access to lonafarnib for children with Progeria prior to potential regulatory submission for approval of lonafarnib.
“PRF is grateful to our donors and volunteers who have enabled us to discover lonafarnib’s effect on survival,” said Meryl Fink, President and Executive Director of PRF. “We are thrilled to partner with Eiger as the company prepares to approach the FDA regarding potential drug approval for lonafarnib in Progeria. Today marks an exciting step forward for families living with Progeria as our partnership with Eiger assures a reliable source of lonafarnib at no cost to patients. Our new agreement signals real progress and hope for all of us in the Progeria community who have worked together to tackle this rapid-aging disease,” said Ms. Fink.
“Continued patient access to lonafarnib was the fundamental motivation for this agreement with PRF,” said David Cory, President and CEO of Eiger. “Eiger will provide lonafarnib for ongoing clinical trials and expanded access in Progeria and work together with PRF to seek regulatory guidance on a pathway toward regulatory approval of lonafarnib for use in children with Progeria.”
About The Progeria Research Foundation
The Progeria Research Foundation (PRF) was founded in 1999 by the family of Sam Berns in response to the complete lack of attention to, and progress being made in, Progeria research. The original mission: to discover the cause, treatments and cure for Progeria. In 2003 the PRF Genetics Consortium discovered the Progeria gene, a collaboration led by Dr. Francis Collins, then Director of the National Human Genome Research Institute, and who is currently Director of the National Institutes of Health (NIH). Today, PRF continues to be the only organization in the world solely dedicated to finding treatments and the cure for Progeria and its aging-related conditions, including heart disease. The organization fills a void, taking these children out of the background where they had been for more than 100 years and putting them and Progeria at the forefront of scientific efforts. For more information and to donate to PRF, please visit www.progeriaresearch.org.
About Eiger BioPharmaceuticals, Inc.
Eiger BioPharmaceuticals (NASDAQ: EIGR) is a clinical-stage biopharmaceutical company focused on the development and commercialization of targeted therapies for rare diseases. Eiger is committed to translational innovation and the development of well-characterized drugs acting on newly identified or novel targets. Eiger’s mission is to systematically reduce the time and cost of the drug development process to more rapidly deliver important medicines to patients with rare diseases. Eiger’s lead program evaluating lonafarnib in Hepatitis Delta Virus (HDV) infection is moving into Phase 3 with a single, pivotal trial planned to initiate by the end of the year. For additional information about Eiger and its clinical programs, please visit www.eigerbio.com.
JAMA reference: Gordon et. al., Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome, JAMA, April 24, 2018 Volume 319, Number 16 and accompanying editorial.
Accompanying Editorial: Hisama and Oshima, Precision Medicine and Progress in the Treatment of Hutchinson-Gilford Progeria Syndrome.
# # #
Media Contact:
Spectrum Science Communications
John J. Seng
jjs@spectrumscience.com
P: (+1) 202 587-2555